Abstract
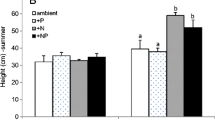
Salt marshes along the northeastern coast of the United States are increasingly subject to changes in hydrology and enrichment with nitrogen as a result of human activities. We conducted a greenhouse experiment to determine the response of Phragmites australis, Spartina alterniflora, and their root-associated microbial communities to these environmental perturbations. Two sets of treatments were compared: 1) saturated versus drained hydrology under low N enrichment and 2) low versus high N enrichment under drained hydrologic conditions. Unvegetated sediments were planted with either Phragmites australis or Spartina alterniflora, and after one growing season, sediment characteristics, macrophyte biomass, and sediment microbial community structure, as described by phospholipid fatty acids (PLFAs), were analyzed. Under all conditions tested, Spartina root production was significantly greater than Phragmites. While Spartina invested more biomass in roots, Phragmites invested proportionally more biomass in shoots and rhizomes, and Phragmites response to drained hydrology or to an increase in N also differed from that of Spartina. Under N enrichment, the rate of Phragmites stem production doubled, and under drained conditions the ratio of Phragmites shoot:root biomass increased, while Spartina biomass ratios remained unchanged. Although Spartina root biomass was significantly greater than that of Phragmites, under drained conditions the Spartina sediment PLFA diversity was significantly lower than the PLFA diversity in both Phragmites and unvegetated sediments. Under saturated conditions, vegetated sediments exhibited greater PLFA diversity, while no diversity differences were seen in unvegetated sediments under the two hydrologic conditions. Six PLFAs were responsible for 80% of the separation seen within the Principal Components Analysis ordination space. Significant differences in these PLFAs were due to hydrology when comparing saturated vs. drained sediments, and predominantly due to the plant species when comparing N treatments under drained conditions. Our results suggest that macrophyte root association can influence the structure of estuarine sediment microbial communities, but that saturated hydrological conditions may override the plant influences.
Similar content being viewed by others
Literature Cited
Armstrong, J., W. Armstrong, P. M. Beckett, J. E. Halder, S. Lythe, R. Holt, and A. Sinclair. 1996. Pathways of aeration and the mechanisms and beneficial effects of humidity- and Venturi-induced convections in Phragmites australis (Cav.) Trin. Ex Steud. Aquatic Botany 54: 177–97.
Bagwell, C. E., M. Dantzler, P. W. Bergholz, and C. R. Lovell. 2001. Host-specific ecotype of rhizoplane diazotrophs of the perennial glasswort Salicornia virginica and selected salt marsh grasses. Aquatic Microbial Ecology 23: 293–300.
Bagwell, C. E. and C. R. Lovell. 2000. Microdiversity of culturable diazotrophs from the rhizoplanes of the salt marsh grasses Spartina alterniflora and Juncus roemerianus. Microbial Ecology 39: 128–36.
Bagwell, C. E., Y. M. Piceno, A. Ashburne-Lucas, and C. R. Lovell. 1998. Physiological diversity of the rhizosphere diazotroph assemblages of selected salt marsh grasses. Applied and Environmental Microbiology 64: 4276–82.
Barko, J. W., D. Gunnison, and S. R. Carpenter. 1991. Sediment interactions with submersed macrophyte growth and community dynamics. Aquatic Botany 41: 41–65.
Bart, D. and J. M. Hartman. 2001. Environmental constraints on early establishment of Phragmites australis in salt marshes. Wetlands 22: 201–13.
Bergholz, P. W., C. E. Bagwell, and C. R. Lovell. 2001. Physiological diversity of rhizoplane diazotrophs of the salt meadow cordgrass, Spartina patens: implications for host specific ecotypes. Microbial Ecology 42: 466–73.
Bertness, M. D., P. J. Ewanchuk, and B. R. Silliman. 2002. Anthropogenic modification of New England salt marsh landscapes. Proceedings of the National Academy of Science 99: 1395–98.
Burke, D., E. Hamerlynck, and D. Hahn. 2002. Interactions among plant species and microorganisms in salt marsh sediments. Applied and Environmental Microbiology 68: 1157–64.
Chambers, R. M. 1997. Porewater chemistry associated with Phragmites and Spartina in a Connecticut tidal marsh. Wetlands 17: 360–67.
Chambers, R. M., L. A. Meyerson, and K. Saltonstall. 1999. Expansion of Phragmites australis into tidal wetlands of North America. Aquatic Botany 64: 261–73.
Chambers, R. M., D. T. Osgood, D. J. Bart, and F. Montalto. 2003. Phragmites australis invasion and expansion in tidal wetlands: interactions among salinity, sulfide, and hydrology. Estuaries 26: 398–406.
Coleman, D. C. and D. A. Crossley, Jr. 2003. Water as a constituent of soil. p. 1–5. In D. C. Coleman, D. A. Crossley, Jr., and P. F. Hendrix (eds.) Fundamentals of Soil Ecology. Academic Press, Burlington, MA, USA.
Dai, T. and R. G. Wiegert. 1997. A field study of photosynthetic capacity and its response to nitrogen fertilization in Spartina alterniflora. Estuarine, Coastal and Shelf Science 45: 273–83.
de la Cruz, A. A., C. T. Hackney, and N. Bhardwaij. 1989. Temporal and spatial patterns of redox potential (Eh) in three tidal marsh communities. Wetlands 9: 181–90.
de Souza, M. P. and D. C. Yoch. 1997. Spartina alterniflora dieback recovery correlates increased acetylene reduction activity in saltmarsh sediments. Estuarine, Coastal and Shelf Science 45: 547–55.
Drake, H. L. 1994. Acetogenesis. Chapman and Hall, New York, NY, USA.
Farnsworth, E. J. and L. A. Meyerson. 2003. Comparative ecophysiology of four wetland plant species along a continuum of invasiveness. Wetlands 23: 750–62.
Gandy, E. L. and D. C. Yoch. 1988. Relationship between nitrogen-fixing sulfate reducers and fermenters in salt marsh sediments and roots of Spartina alterniflora. Applied and Environmental Microbiology 54: 2031–36.
Hellings, S. E. and J. L. Gallagher. 1992. The effects of salinity and flooding on Phragmites australis. Journal of Applied Ecology 29: 41–49.
Herbert, R. A. 1999. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiology Reviews 23: 563–90.
Hines, M. E., R. S. Evans, B. R. S. Genthner, S. G. Willis, S. Friedman, J. N. Rooney-Varga, and R. Devereux. 1999. Molecular phylogenetic and biogeochemical studies of sulfate-reducing bacteria in the rhizosphere of Spartina alterniflora. Applied and Environmental Microbiology 65: 2209–16.
Hocking, P. 1989. Seasonal dynamics of production and nutrient accumulation and cycling by Phragmites australis (Cav.) Trin. Ex Stuede in a nutrient-enriched swamp in inland Australia. I. Whole Plants. Australian Journal of Marine and Freshwater Resources 40: 421–44.
Howes, B. L., J. W. H. Dacey, and D. D. Goehringer. 1986. Factors controlling the growth form of Spartina alterniflora: feedbacks between above-ground production, sediment oxidation, nitrogen and salinity. Journal of Ecology 74: 881–98.
Howes, B. L., R. W. Howarth, J. M. Teal, and I. Valiela. 1981. Oxidation-reduction potentials in a salt marsh: spatial patterns and interactions with primary production. Limnology and Oceanography 26: 350–60.
Hunt, R. 1990. Basic Growth Analysis. Unwin Lyman, Ltd., London, UK.
Kadlec, R. H. and R. L. Knight. 1996. Treatment Wetlands. Lewis Publishers, New York, NY, USA.
Klepac-Ceraj, V., M. Bahr, B. C. Crump, A. P. Teske, J. E. Hobbie, and M. F. Polz. 2004. High overall diversity and dominance of microdiverse relationships in salt marsh sulphate-reducing bacteria. Environmental Microbiology 6: 686–98.
Koch, M. S., I. A. Mendelssohn, and K. L. McKee. 1990. Mechanism for the hydrogen sulfide-induced growth limitation in wetland macrophytes. Limnology and Oceanography 35: 399–408.
Kohzu, A., K. Matsui, T. Yamada, A. Sugimoto, and N. Fugita. 2003. Significance of rooting depth in mire plants: evidence from natural 15N abundance. Ecological Research 18: 257–66.
Koretsky, C. M., P. Van Cappellen, T. J. DiChristina, J. E. Kostka, K. L. Lowe, C. M. Moore, A. N. Roychoudhury, and E. Viollier. 2005. Salt marsh pore water geochemistry does not correlate with microbial community structure. Estuarine Coastal and Shelf Science 62: 233–51.
Kostka, J. E., A. Roychoudhury, and P. Van Cappellen. 2002. Rates and controls of anaerobic respiration across spatial and temporal gradients in saltmarsh sediments. Biogeochemistry 60: 49–76.
LaRocque, J. R., P. W. Bergholz, C. E. Bagwell, and C. R. Lovell. 2004. Influence of host plant-derived and abiotic environmental parameters on the composition of the diazotroph assemblage associated with roots of Juncus roemerianus. Antonie van Leeuwenhock 68: 249–61.
Leaphart, A. B., M. J. Friez, and C. R. Lovell. 2003. Formyltetrahydrofolate synthetase sequences from salt marsh plant roots reveal a diversity of acetogenic bacteria and other bacterial functional groups. Applied and Environmental Microbiology 69: 693–96.
Levine, J. M., J. S. Brewer, and M. D. Bertness. 1998. Nutrients, competition and plant zonation in a New England salt marsh. Journal of Ecology 86: 285–92.
Li, L., D. P. Horn, and A. J. Baird. 2006. Tide induced variations in surface temperature and water-table depth in the intertidal zone of a sandy beach. Journal of Coastal Research 22: 1370–81.
Lovell, C. R., C. E. Bagwell, M. Czákó, L. Márton, Y. M. Piceno, and D. B. Ringelberg. 2001. Stability of a rhizosphere microbial community exposed to natural and manipulated environmental variability. FEMS Microbial Ecology 38: 69–76.
Mendelssohn, I. A., K. L. McKee, and W. H. Patrick, Jr. 1981. Oxygen deficiency in Spartina alterniflora roots: metabolic adaptation to anoxia. Science 214: 439–41.
Mendelssohn, I. A., B. K. Sorrell, H. Brix, H. Schierup, B. Lorenzen, and E. Maltby. 1999. Controls on soil cellulose decomposition along a salinity gradient in a Phragmites australis wetland in Denmark. Aquatic Botany 64: 381–98.
Meyerson, L. A., K. Saltonstall, L. Windham, E. Kiviat, and S. Findlay. 2000. A comparison of Phragmites australis in freshwater and brackish marsh environments in North America. Wetland Ecological Management 9: 89–103.
Minchinton, T. E. and M. D. Bertness. 2003. Disturbance-mediated competition and the spread of Phragmites australis in a coastal marsh. Ecological Applications 13: 1400–16.
Nielsen, L. B., K. Finster, D. T. Welsh, A. Donelly, R. A. Herbert, R. deWit, and B. A. Lomstein. 2001. Sulphate reduction and nitrogen fixation rates associated with roots, rhizomes and sediments from Zostera noltii and Spartina maritime meadows. Environmental Microbiology 3: 63–71.
Niering, W. A. and R. S. Warren. 1980. Vegetation patterns and processes in a New England salt marsh. BioScience 30: 301–07.
Nuttle, W. K. 1988. The extent of lateral water movement in the sediments of a New England salt marsh. Water Resources Research 24: 2077–85.
Otto, S., P. M. Groffman, S. E. G. Findlay, and A. E. Arreola. 1999. Invasive plant species and microbial processes in a tidal freshwater marsh. Journal of Environmental Quality 28: 1252–57.
Piceno, Y. M. and C. R. Lovell. 2000a. Stability in natural bacterial communities: I. nutrient addition effects on rhizosphere diazotroph assemblage composition. Microbial Ecology 39: 32–40.
Piceno, Y. M. and C. R. Lovell. 2000b. Stability in natural bacterial communities: II. plant resource allocation effects on rhizosphere diazotroph assemblage composition. Microbial Ecology 39: 41–48.
Piceno, Y. M., P. A. Noble, and C. R. Lovell. 1999. Spatial and temporal assessment of diazotroph assemblage composition in vegetated salt marsh sediments using denaturing gradient gel electrophoresis analysis. Microbial Ecology 38: 157–67.
Ratledge, C. and S. G. Wilkinson. 1988. Microbial Lipids, Volumes 1–2. Academic Press, San Diego, CA, USA.
Ravit, B., J. G. Ehrenfeld, and M. M. Häggblom. 2003. A comparison of sediment microbial communities associated with Phragmites australis and Spartina alterniflora in two brackish wetlands of New Jersey. Estuaries 26: 465–74.
Ravit, B., J. G. Ehrenfeld, and M. M. Häggblom. 2005. Salt marsh rhizosphere affects microbial biotransformation of the widespread halogenated contaminant tetrabromobisphenol A (TBBPA). Soil Biology and Biochemistry 37: 1049–57.
Ravit, B., J. G. Ehrenfeld, and M. M. Häggblom. 2006. Effects of vegetation on root associated microbial communities: a comparison of disturbed versus undisturbed estuarine sediments. Soil Biology and Biochemistry 38: 2359–71.
Rice, D., J. Rooth, and J. C. Stevenson. 2000. Colonization and expansion of Phragmites australis in upper Chesapeake Bay tidal marshes. Wetlands 20: 280–99.
Rickey, M. A. and R. C. Anderson. 2004. Effects of nitrogen addition on the invasive grass Phragmites australis and a native competitor Spartina pectinate. Journal of Applied Ecology 41: 888–96.
Roman, C. T., W. A. Niering, and R. S. Warren. 1984. Salt marsh vegetation change in response to tidal restriction. Environmental Management 8: 141–50.
Roman, C. T., K. B. Raposa, S. C. Adamowicz, M.-J. James-Pirri, and J. G. Catena. 2002. Quantifying vegetation and nekton response to tidal restoration of a New England salt marsh. Restoration Ecology 10: 450–60.
Rooth, J. E. and J. C. Stevenson. 2000. Sediment deposition patterns in Phragmites australis communities: implications for coastal areas threatened by rising sea-level. Wetlands Ecology and Management 8: 173–83.
Saltonstall, K. 2002. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proceedings of the National Academy of Science 99: 2445–49.
Saltonstall, K. and J. C. Stevenson. 2007. The effect of nutrients on seedling growth of native and introduced Phragmites australis. Aquatic Botany: (in press).
Šantrůčková, H., T. Picek, M. Šimek, V. Bauaer, J. Kopecky, L. Pechar, J. Lukanská, and H. Čížková. 2001. Decomposition processes in soil of a healthy and a declining Phragmites stand. Aquatic Botany 69: 217–34.
Sinicrope, T. L., P. G. Hine, R. S. Warren, and W. A. Niering. 1990. Restoration of an impounded salt marsh in New England. Estuaries 13: 25–30.
Sundareshwar, P. V., J. R. Morris, E. K. Koepfler, and B. Fornwalt. 2003. Phosphorus limitation of coastal ecosystem processes. Science 299: 563–66.
Tyler, A. C., T. A. Mastroniccola, and K. J. McGlathery. 2003. Nitrogen fixation and nitrogen limitation of primary production along a natural marsh chronosequence. Oecologia 136: 431–38.
Valiela, I. and J. M. Teal. 1974. Nutrient limitation in salt marsh vegetation. p. 547–63. In R. J. Reimold and W. H. Queen (eds.) Ecology of Halophytes. Academic Press, New York, NY, USA.
White, D. C., W. M. Davis, J. S. Nickels, J. D. King, and R. J. Bobbie. 1979. Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 40: 51–62.
Wigand, C., G. B. Thursby, R. A. McKinney, and A. F. Santos. 2004. Response of Spartina patens to dissolved inorganic nutrient additions in the field. Journal of Coastal Research 45: 134–49.
Windham, L. and J. G. Ehrenfeld. 2003. Net impact of a plant invasion on nitrogen-cycling processes within a brackish tidal marsh. Ecological Applications 13: 883–97.
Windham, L. and R. G. Lathrop, Jr. 1999. Effects of P.australis invasion on aboveground biomass and soil properties in brackish tidal marsh of Mullica River, NJ. Estuaries 22: 927–35.
Windham-Myers, L. 2005. Dissolved inorganic nitrogen pools and surface flux under different brackish marsh vegetation types, common reed (Phragmites australis) and salt hay (Spartina patens). Biogeochemistry 75: 289–304.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ravit, B., Ehrenfeld, J.G., Häggblom, M.M. et al. The effects of drainage and nitrogen enrichment on Phragmites australis, Spartina alterniflora, and their root-associated microbial communities. Wetlands 27, 915–927 (2007). https://doi.org/10.1672/0277-5212(2007)27[915:TEODAN]2.0.CO;2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1672/0277-5212(2007)27[915:TEODAN]2.0.CO;2